Medical equipment is expected to work without fail. Bel manufactures its power supplies to the most rigorous standards so that system designers can use our products with the utmost confidence.
Standards have been adopted for nearly every aspect of medical electronics. They start at the highest conceptual level, dictating how medical data is handled (HIPAA), but then drill down into details such as how software must behave (ISO 62304), and what quality control means in manufacturing (ISO 13485). Another standard, IEC 60601, includes a comprehensive set of technical specifications for the safety and effectiveness of medical electrical hardware, including everything from system-level performance to power supplies.
Technological innovation is perpetual, affecting expectations of performance and reliability, and compelling constant revisions in standards. ISO schedules a review of its medical standards every five years to determine if updates are required.
IEC 60601-1 has been updated periodically, most recently in December of 2023.
Changes in IEC 60601 to Date
The original version of the IEC 60601 standard addressing medical devices was published in 1977. The 2nd edition, published in 1988, broadened the focus of the original standard to encompass safety within the vicinity of a patient. The 3rd edition, released in 2005, embraced the concept of “means of protection” (MOP) and took a more expansive view of the safety of medical equipment considering both patients and equipment operators.
Figure 1: Introduction of MOP Classifications.
| MOP | MOOP | MOPP |
| Means of Protection | Means of Operator Protection | Means of Patient Protection |
Though the next update, IEC 60601-1 3.1 introduced in 2012, was not a major revision, it still introduced a number of substantial changes, addressing numerous ambiguities arising from evolving medical equipment technology.
Note that within IEC 60601-1, there are two types of subsidiary standards, called collateral and particular standards. Collateral standards include, for example, IEC 60601-1-3, which covers radiation protection for diagnostic x-ray systems, 60601-1-9 which relates to environmental design, and 60601-1-11 which pertains to home healthcare equipment. An example of a particular standard, meanwhile, is IEC 60601-2-16, which covers blood dialysis and filtration equipment.
IEC 60601-1-3 |
IEC 60601-1-9 |
IEC 60601-1-11 |
IEC 60601-2-16 |
Another update in 2014, involved the collateral standard IEC 60601-1-2, “Electromagnetic disturbances – Requirements and tests.”
The New Revision in IEC 60601
The most recent revision of IEC 60601-1 was given the designation IEC 60601-1 version 3.2. That marks it as an amendment (as opposed to a major new update), but this update also involves some substantial changes, including replacing entire sections of the standard. Version 3.2 clarifies and supplements provisions concerning potential electrical, thermal, mechanical, and functional hazards, again considering both patients and equipment operators.
Some of the changes, among them the changes to Clause 8 (Protection Against Electrical Hazards), refer specifically to power supplies.
While the IEC has been planning a major new revision for years, it still has no target date for publishing the 4th Edition.
A De Facto Requirement
While compliance with IEC 60601 is not mandatory everywhere around the world, it is required in the U.S. and in Europe. As a practical matter, that makes it a global prerequisite for bringing new medical devices to market.
That said, it is important for medical equipment manufacturers to be aware that there are local variations that they might have to conform to, depending on where they choose to do business. EN 60601-1 in Europe and CSA 60601-1 in Canada are largely identical to the IEC standard, but there are other markets that have adopted versions of the standard with consequential deviations.
Manufacturers should also be aware that different markets take varying amounts of time to adjust to new updates, and that is certainly what is happening with the relatively new 60601-1 3.2. In fact, in most markets, the process of harmonizing 3.1 with 3.2 has only just begun, if it has started at all. As a practical matter, products that conform to 3.1 are still manufactured to the highest standards being applied today, and will continue to be approved for use in medical systems for years to come.
It is recommended that medical equipment manufacturers become conversant with all of the changes and investigate how they might apply to systems currently in development. Conforming to changes can incur new expenses associated with verification and testing processes and procedures. It can also occasionally compel manufacturers to make design changes, which can be costly. So, it is best to determine what steps will be necessary at the earliest possible stage in the development process.
IEC 60601-1 and Power Supplies
Each generation of products tends to get more complex, compounding potential test cases, permutations, and combinations in both normal and abnormal operating modes.
While power supplies by themselves are not medical devices and are not directly covered by IEC 60606-1, they are acknowledged in the standards to be critical elements in the safe operation of medical equipment.
The voltages and currents provided by both ac-dc power supplies and dc-dc converters means there is an inherent hazard if not properly managed.
Figure 2: Medical staff using electronic equipment.
Safety Within the Vicinity (2nd Edition)
In the context of safe operation of medical equipment, the 2nd edition of IEC 60601-1 established risk guidelines that applied when a device was within a 6-foot radius of the patient, referred to as the “patient vicinity.”
Figure 3: Patient vicinity.
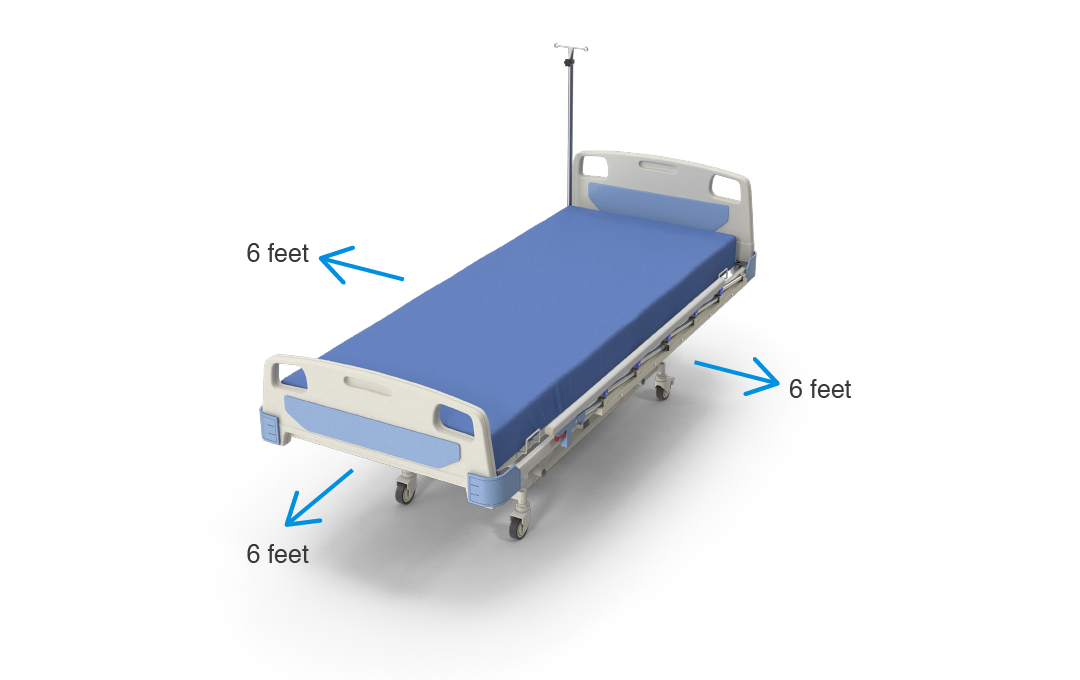
Three use categories of increasing severity were defined:
- Type B (body) equipment operates without patient contact. Examples include x-ray machines, hospital beds, LED operating lighting, and MRI scanners.
- Type BF (body floating) equipment makes physical contact with the patient. Examples include blood pressure monitors, ultrasound equipment, and thermometers.
- Type CF (cardiac floating) makes physical contact with the heart. Examples include defibrillators and dialysis machines.
The standard sets applicable levels of isolation, insulation, creepage, clearance, and leakage in each class.
MOP and Power Supplies (3rd Edition)
The concept of means of protection (MOP) introduced in the 3rd edition recognizes that there’s a difference in the potential hazards that patients and medical equipment operators might experience. MOP gets divided into two categories: means of operator protection (MOOP) and means of patient protection (MOPP).
Table 1: Isolation, creepage, and insulation requirements for MOP classifications.
| Classification | Isolation | Creepage | Insulation |
|---|---|---|---|
| One MOOP | 1500 VAC | 2.5 mm | Basic |
| Two MOOP | 3000 VAC | 5.0 mm | Double / Reinforced |
| One MOPP | 1500 VAC | 4.0 mm | Basic |
| Two MOPP | 4000 VAC | 8.0 mm | Double / Reinforced |
What follows are examples of what these distinctions mean in practice. For 2nd edition Type B use, the standard requires a device to have two isolation barriers to meet MOP requirements. However, a non-medical rated supply may be satisfactory as long as it has reduced leakage currents below 500 µA – this meets what the 3rd edition refers to as one MOPP protection. A 2nd edition Type BF application needs to meet the 3rd edition’s two MOOP and one MOPP requirements, however. Meanwhile, a 2nd edition Type CF not only requires an IEC 60601-1 qualified supply but must ensure an additional isolation barrier between the supply and the part that touches the patient.
Analyzing Risk
The 3rd edition standard specifically calls out the Risk Management Process described in ISO 14971 that includes a risk management file where identifiable fault conditions are identified and assessed.
It also demands greater interaction between the equipment manufacturer and the test laboratory. This distinguishes between the criteria established by the risk analysis and the test processes used to demonstrate compliance.
The 3.1 edition introduced in 2012 clarifies provisions in the standard that the industry found ambiguous. This update includes almost 500 changes and clarifications across a spectrum of subjects, including essential performance, risk management, mechanical testing, temperature testing, and humidity testing. The amended standard also defines several new specifications for mechanical and electrical hazards.
What's Next
Given that the global market is still digesting the revisions in 60601-3.2, there are no major updates likely in the near future. Most of the activity regarding IEC 60601 will be market-bymarket harmonization of 3.1 and 3.2.
Minor revisions are almost always in the pipeline, and that remains true today. An example is IEC 60601-1-2 4th edition – which pertains to collateral standards within IEC 60601. This update considers immunity in terms of “intended use environments.”
It will also no longer include the “life-supporting” equipment category. What prompted these changes is that it is becoming harder to define what qualifies for that category as more medical equipment gets installed in environments that are not hospitals, including dental offices, homes, and factories.
Figure 4: Typical home medical products.
From a societal standpoint, it is quite significant that more people can safely use medical equipment in more places, but from a design and manufacturing standpoint, these changes are relatively minor.
Navigating Compliance Challenges
The IEC 60601 standard is complex, continuously evolving, and has regional variations. This makes compliance inherently challenging, even before having to contend with the ambiguities that arise as technology evolves.
Documentation of compliance adds to the challenge. Simply shipping a medical device with basic documentation to a certification lab is no longer adequate. Comprehensive, carefully structured documentation is needed for the design analysis, the design process, and the design rationale with explanations for why certain elements were or were not included or undertaken. The increasing emphasis on risk assessment and considerations about use environments adds to this burden.
In order to help ease the compliance process for medical device designers, Bel offers a comprehensive line of external and embedded medical power supplies ranging from 6 watts to 1000 watts that have been certified to IEC 60601 Edition 3.1 two MOPP safety standards and 4th edition EMC standards.
Figure 5: Medical staff using electronic equipment.
See Bel's selection of external and embedded medical power supplies.
Learn More